Regulatory Doctor
Medical and Health Tips (MHT) |
33 videos |
Updated 2 weeks ago
How Regulatory Doctor Supports Drug Development and Medical Device Success
Navigating the FDA approval process for drugs and medical devices can be challenging in today’s complex regulatory environment. From clinical development to postmarket surveillance, companies encounter significant hurdles that require strategic planning, technical expertise, and a thorough understanding of evolving regulatory standards.
At Regulatory Doctor, we specialize in streamlining your path to market while ensuring compliance with FDA requirements. Here’s how we address the critical areas in drug development and medical devices:
Drug Development: Comprehensive Support Across the Lifecycle
Clinical Development and Trial Design
Our Solution: We create tailored clinical trial strategies aligned with FDA expectations, ensuring efficient progression through all phases, including adaptive protocols and IND submissions.
Our Edge: By aligning with the FDA’s Critical Path Initiative, we enhance trial efficiency and endpoint selection for regulatory success.
Chemistry, Manufacturing, and Controls (CMC)
Our Solution: We cover all aspects of drug substance and product development, ensuring compliance with ICH and FDA standards.
Our Edge: Our expertise in stability studies, Quality by Design (QbD) implementation, and comparability assessments for APIs—both synthetic and biologically derived—sets us apart.
Regulatory Strategy and Submissions
Our Solution: We provide clear roadmaps for navigating NDA, ANDA, and BLA submissions, focusing on accelerating approvals.
Our Edge: By mitigating risks like Refuse-to-File (RTF) actions and leveraging designations such as Orphan Drug or Breakthrough Therapy, we position our clients for success.
Biologics and Advanced Therapies
Our Solution: We guide biologics through complex regulatory pathways, including CAR-T therapies and biosimilars.
Our Edge: Our specialization in comparability strategies and Type A/B/C meeting preparation ensures smooth transitions from preclinical to clinical phases.
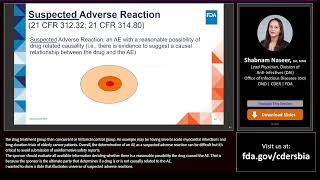
Pharmacovigilance and Risk Management
Our Solution: We develop robust safety frameworks for effective management of post-market risks.
Our Edge: Our expertise in creating REMS plans and adverse event reporting systems ensures compliance and patient safety.
Medical Devices: Navigating Innovation and Compliance
Premarket Submissions
Our Solution: We streamline 510(k), PMA, and De Novo submissions, ensuring readiness for FDA review.
Our Edge: Our proactive gap analyses and facilitation of FDA pre-submission meetings minimize delays and regulatory hurdles.
Quality Management Systems (QMS) and Compliance
Our Solution: We enhance QMS frameworks and prepare clients for FDA inspections.
Our Edge: From CAPA system implementation to responding to FDA Form 483 observations, we provide actionable solutions.
Combination Products
Our Solution: We simplify regulatory complexities for products that combine drugs, devices, and biologics.
Our Edge: Our close coordination with the FDA’s Office of Combination Products ensures a streamlined approval process.
Software as a Medical Device (SaMD)
Our Solution: We guide digital health innovators through FDA’s SaMD regulations and cybersecurity standards.
Our Edge: Our expertise in AI/ML-enabled device submissions helps clients meet the FDA’s evolving expectations.
Postmarket Surveillance and Reporting
Our Solution: We build effective systems for managing adverse events and recalls.
Our Edge: From MDR compliance to recall strategies, we help clients mitigate risks and maintain compliance.
Why Choose Regulatory Doctor?
At Regulatory Doctor, we combine technical expertise, regulatory insights, and strategic planning to tackle the most challenging aspects of FDA approval. Whether you’re a biotech startup or an established pharmaceutical company, our tailored solutions empower you to overcome hurdles, reduce time-to-market, and achieve compliance with confidence.
Let us be your partner in success. Contact Regulatory Doctor today to learn how we can help you navigate the FDA’s regulatory landscape and bring your innovations to market.
For more information, please visit us at regulatorydoctor.us